News
Read the latest news, case studies & event reports from our Flow Community! Want to stay up to date? Sign-up for our newsletter & a few times per year we will e-mail you interesting articles, developments, up-coming event info!
Pfizer Showcase Industrial-scale Continuous Manufacturing!
30 May 2021
In the midst of a global pandemic, Pfizer have used Continuous Manufacturing to accelerate the production of the Pfizer-BioNTech COVID-19 vaccine! By developing a continuous technique to control encapsulation of mRNA in lipid nanoparticles (LNP’s), Pfizer have been able to rapidly produce a stable formulation that is distributed & administered globally.
Watch the CNN news report by Dr Sanjay Gupta to see how parallelisation (or numbering-up) of 100 static mixers, called an impingement jet mixer (IJM), have been used to increase vaccine productivity at their site in Kalamazoo (US) to 100 million doses/month! Not satisfied with using modular production techniques for manufacturing, Pfizer also used pre-fabricated modules to fast track building of the facility in which the vaccine is produced! Read more on small, modular plants.
If people question can ‘Continuous Manufacturing be used for the production of Pharmaceuticals?’, remember the part it had to play in getting 540 million doses of the Pfizer-BioNTech COVID-19 vaccine (as of 2021-05-22) into the hands of healthcare workers in over 90 Countries!
Pfizer’s work is not the only example of continuous LNP’s development! Just last month, Researchers at Purdue University (USA) published their preliminary findings into the development of an alternative treatment for bladder cancer based on an RWFV-targeted stabilised lipid nucleic acid nanoparticle link.
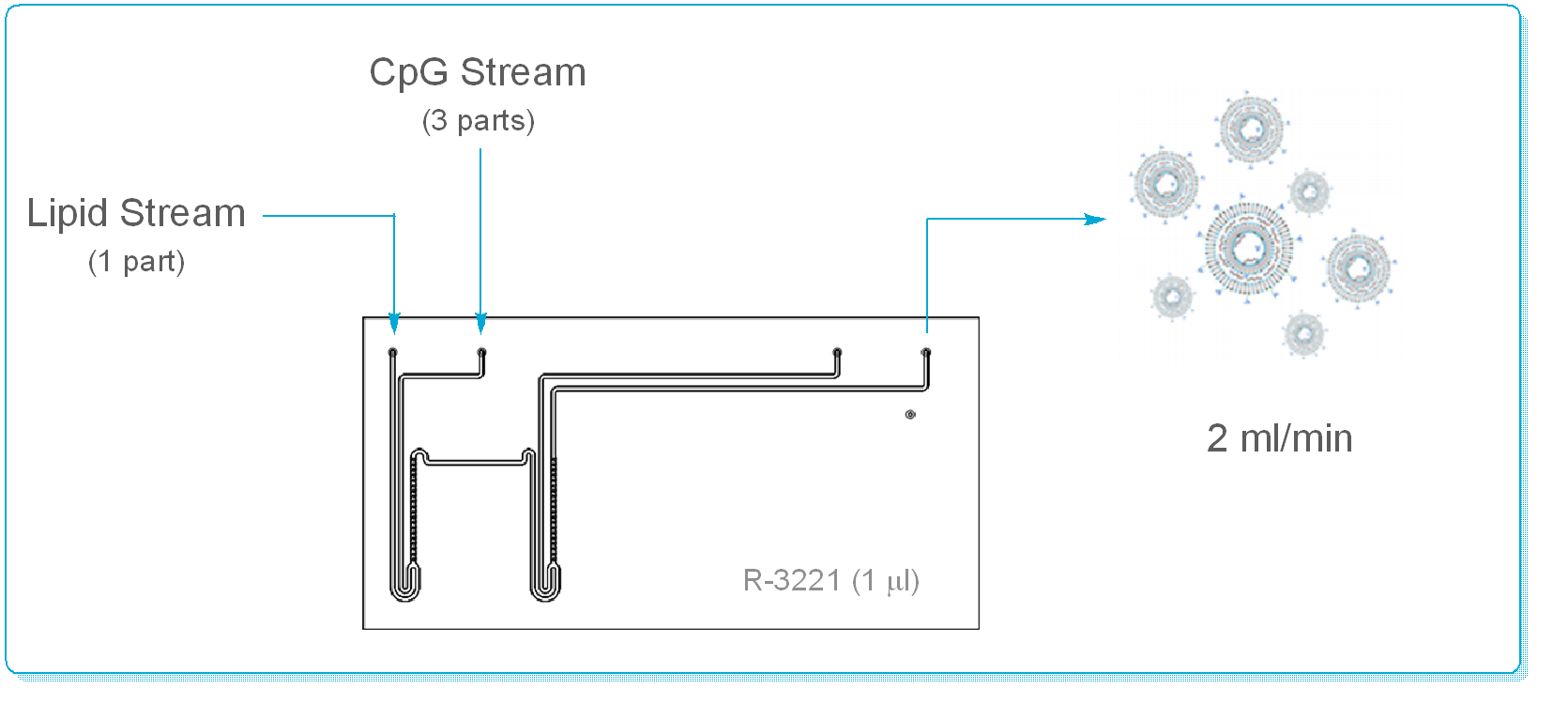
Using a Labtrix® glass flow reactor (Reaction Volume = 1 ml), Thompson & co-workers were able to demonstrate exquisite control over LNP size & size distribution, together with validation of efficient delivery of CpG by the LNP. Early indications show that these pH sensitive CpG LNP’s demonstrate an enhanced immunostimulatory response cf. pH insensitive LNP’s & may be a highly efficient, low risk alternative to Bacillus Calmette Guerin (BCG) immunotherapy.
By preparing the LNP’s under continuous flow conditions, the Group were able to:
- Tune the N/P ratio without affecting encapsulation efficiency
- Reproducibly control particle size (15 to 40 nm)
- Obtain a narrow particle size distribution (e.g. PDI’s of 0.2 +/- 0.01)
- Remove operator dependency from LNP production
- Demonstrated a technique that can deliver at a 2.9 l/day scale from a 1 ml device!
We look forward to seeing more small molecule & immunotherapy developments from the Purdue Group in the future!


